While researching crystal formation, scientists at New York University discovered a unique rod-shaped crystal previously unrecognized.
Crystals are solid substances composed of particles arranged in repeating patterns.
This self-organization process—often described by researchers as “regulating order from chaos”—was traditionally believed to follow a predictable, classical growth model.
However, they are discovering that crystals can grow through more intricate pathways rather than simply forming building blocks step by step.
To investigate crystal formation, some researchers utilize crystals consisting of small spherical particles known as colloidal particles. These particles are significantly larger than the atoms in other types of crystals.
“Studying colloidal particles allows us to observe the crystallization process at the level of individual particles, which is challenging for atoms due to their small size and rapid movement,” explained Stefano Sacanna, a professor at New York University.
“With colloids, we can visually analyze the shape of the crystal under a microscope.”
To gain insight into how colloidal crystals form, Professor Sacanna and his team conducted experiments observing the behavior of charged colloidal particles under various growth conditions as they transitioned from a salty suspension into a fully developed crystal.
They also conducted thousands of computer simulations to model the growth of the crystals and to explain their experimental observations.
The researchers found that colloidal crystals form through a two-stage process: the initial amorphous mass of particles condenses, followed by a transformation into an ordered crystal structure, resulting in a diverse range of crystal types and shapes.
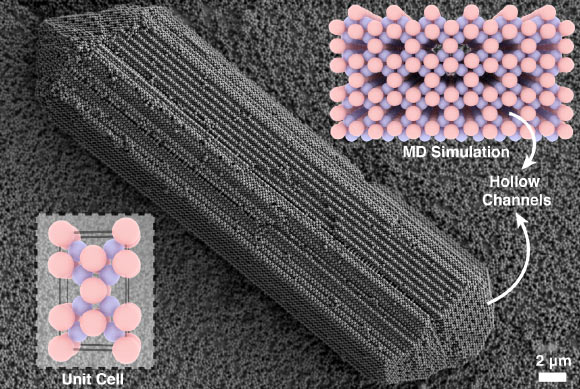
During the experiments, New York University PhD student Shihao Zan encountered a rod-shaped crystal that he could not identify.
While it appeared similar to a previously discovered crystal, detailed examinations revealed differences in the grain combinations and the presence of a hollow channel at the tips of the crystal.
He compared the unknown structures with over 1,000 crystals found in nature but found no match.
By utilizing computer modeling, the researchers were able to simulate the exact crystals, enabling them to study the elongated, hollow shapes more closely.
“This was somewhat perplexing, as crystals are typically dense; however, this one featured empty channels running throughout its length,” remarked Dr. Glenn Hocky from New York University.
“The combined effects of this experiment and simulation led me to realize that this crystal structure had never been documented before,” added Professor Sacanna.
They named the newly identified crystal l3s4 and informally referred to it as “Zangerite” during a lab meeting, reflecting its composition as per Zang’s discovery.
“We study colloidal crystals to replicate the real-world scenarios of atomic crystals, but we never anticipated discovering crystals that wouldn’t resemble those found in nature,” stated Zan.
The discovery of Zangenite holds potential for exploring applications related to hollow low-density crystals and may lead to the identification of more new crystals.
“The channels within Zangenite resemble characteristics found in other materials and may aid in filtering or enclosing internal contents,” Dr. Hocky noted.
“We once thought it was uncommon to find new crystal structures, but we may now be on the verge of discovering additional, yet uncharacterized, structures,” Professor Sacanna added.
A paper detailing this study was published in the journal Nature Communications.
____
S. Zan et al. 2025. Direct observation and control of nonclassical crystallization pathways in binary colloid systems. Nat Commun 16, 3645; doi:10.1038/s41467-025-58959-0
Source: www.sci.news