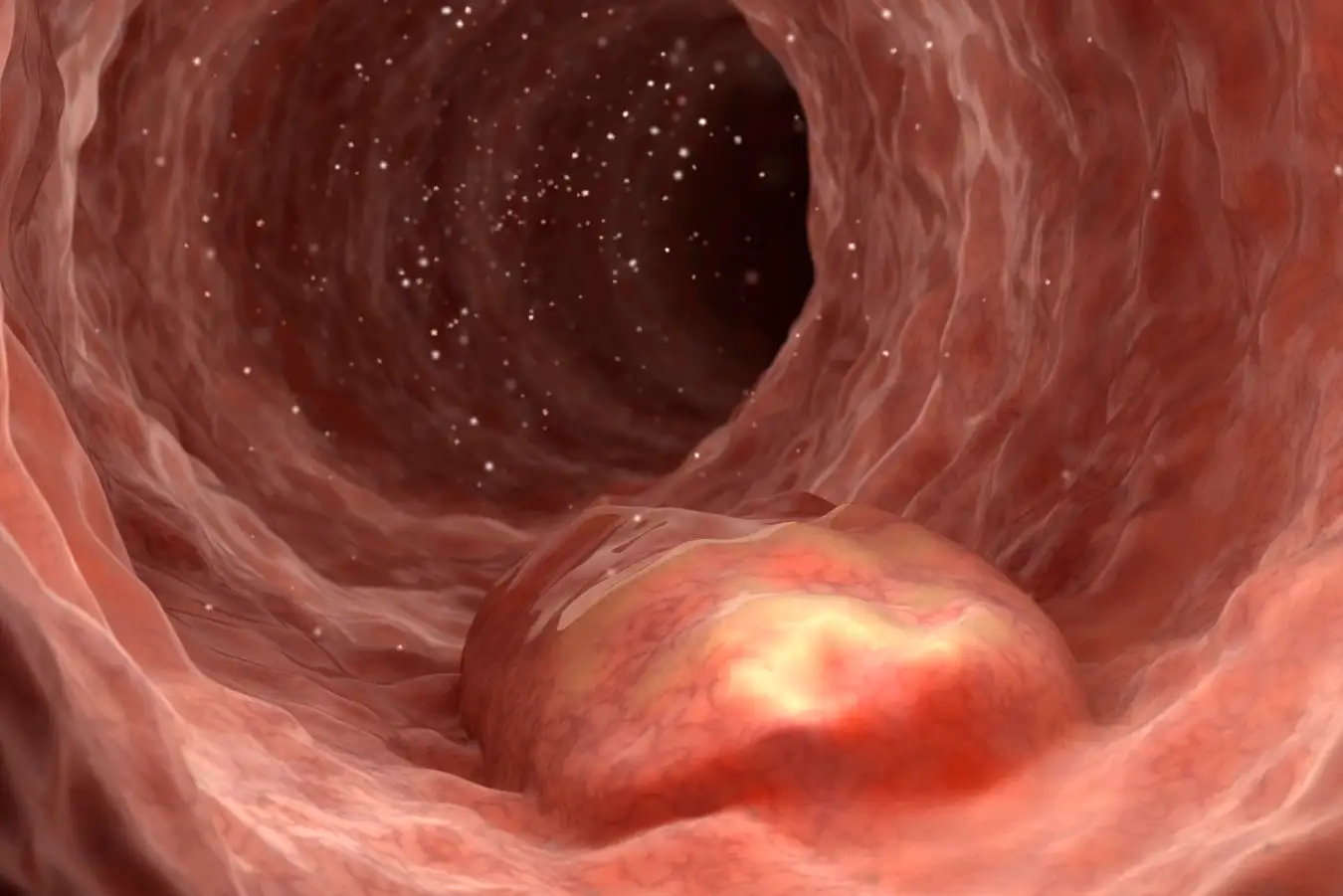
Inflammatory Bowel Disease: Bleeding Wounds Springer Medin/Science Photo Library
Researchers exploring solutions for Inflammatory Bowel Disease (IBD) have drawn surprising inspiration from barnacles.
Inflammatory Bowel Diseases, including Crohn’s disease and ulcerative colitis, typically arise when the immune system mistakenly attacks the intestines, leading to inflammation. Common symptoms encompass diarrhea, significant abdominal pain, weight loss, and gastrointestinal bleeding.
While anti-inflammatory medications like steroids can alleviate symptoms, persistent bleeding may necessitate the use of small metallic clips inserted into the intestine to address the inflammation-induced wounds. However, this procedure carries potential infection risks and may exacerbate the injury.
In pursuit of gentler alternatives, researchers have previously engineered bacteria to generate proteins beneficial for wound healing. Unfortunately, these microorganisms are generally eliminated from the intestines within days and require manual activation with pharmaceuticals, according to Bolin Anne from the Shenzhen Institute of Synthetic Biology in China.
Recently, Ahn and colleagues have genetically modified a benign strain of Escherichia coli that produces protein fragments promoting wound healing upon detecting blood. They also engineered these bacteria to create a type of “cement protein” used by barnacles to adhere to submerged surfaces, envisioned as a “living glue” to fabricate an anti-inflammatory seal over open wounds.
To validate this novel approach, researchers induced intestinal inflammation and scarring in mice. Each subject received either a non-genetically engineered strain, the engineered Escherichia coli, or saline via an anal tube.
After ten days, mice treated with the engineered bacteria exhibited significant weight restoration, and their intestines mirrored the health of uninjured mice. No adverse side effects were recorded in any group.
Similar outcomes were noted when bacteria were administered in tablet form, suggesting potential for oral delivery in human treatment. “This presents a promising, innovative strategy,” states Shaji Sebastian at Hull University in the UK. He indicates that wound healing and inflammation in the mouse intestine is analogous to processes in humans, underscoring the necessity for human trials.
Plans are underway to test this approach in larger animals, including pigs, to assess how long the genetically modified bacteria remain viable in the gut, Ang mentioned. However, due to the necessity for extensive testing to confirm efficacy and safety compared to existing treatments, it may take up to ten years before these solutions could become available in clinics, according to Sebastian.
Topics:
Source: www.newscientist.com