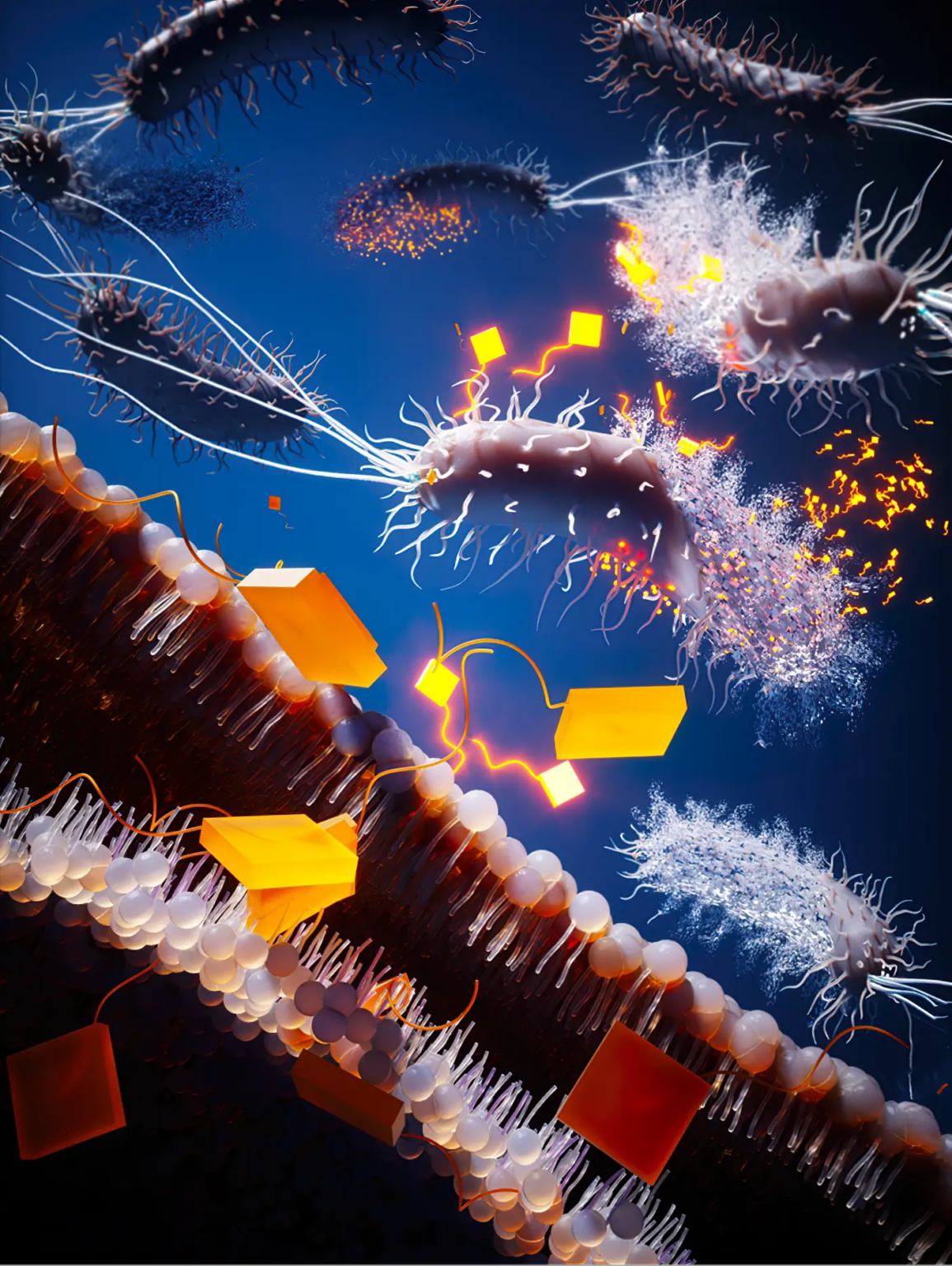
Researchers at Maynooth University have used supramolecular chemistry to discover new molecules to fight drug-resistant bacteria. This new discovery suggests a potential new approach to antibiotic development and has important implications for public health.Credit: Ella Mar Studio
Researchers at Maynooth University have developed a new molecule designed to fight drug-resistant bacteria.
An international team including researchers from Maynooth University has developed a new molecule that has the potential to fight drug-resistant bacteria.
Antimicrobial resistance (AMR) is a phenomenon in which bacteria, viruses, fungi, and parasites evolve over time and become immune to drugs. This resistance makes infections more difficult to cure and increases the risk of prolonged illness and death. With predictions that traditional antibiotics will largely lose their effectiveness by 2050 due to rising AMR levels, finding new ways to eradicate bacteria has become a key scientific priority.
Supramolecular chemistry: the key to fighting AMR
The research leveraged the principles of supramolecular chemistry, a niche scientific field that studies interactions between molecules, to achieve the breakthrough. Most importantly, this study discovered a molecule that is efficient at killing bacteria, yet has very low toxicity to healthy human cells.
New research published in prestigious journal chemistry, in conjunction with World AMR Awareness Week, which will be held from November 18th to 24th. This global campaign, run by the World Health Organization, aims to raise awareness and understanding of AMR in the hope of reducing the emergence and spread of drug-resistant infections.
More than 1.2 million people, and likely millions more, died as a direct result of antibiotic-resistant infections in 2019, according to the most comprehensive estimate to date of the global impact of AMR. The research could pave the way for new approaches to tackling the problem, which kills more people each year than HIV/AIDS or malaria.
Luke Brennan, lead researcher in Maynooth University’s Department of Chemistry, said: “We are discovering new molecules and investigating how they bind to anions, negatively charged chemicals that are very important in the context of the biochemistry of life.” It’s laying a fundamental foundation that could help fight a variety of diseases, from cancer to cystic fibrosis.”
A “Trojan horse” approach to resistant bacteria
The study was based on the use of synthetic ion transporters, and the researchers found that the influx of salts (sodium and chloride ions) into bacteria can trigger a series of biochemical events that lead to bacterial cell death. was demonstrated for the first time. Strains of bacteria that are resistant to currently available antibiotics, such as methicillin-resistant Staphylococcus aureus (MRSA).
Study co-author Dr Robert Hermes from the Kathleen Lonsdale Institute for Human Health at Maynooth University said: “This study shows how our approach, a kind of ‘Trojan horse’ that causes salt influx into cells, can be used to effectively kill resistant bacteria. It eliminates bacteria in a way that counters known bacterial resistance methods.”
Bacteria work hard to maintain a stable concentration of ions within their cell membranes, and when this delicate balance is disrupted, normal cell function is wreaked havoc and the cell is no longer viable.
Elms continued, “These synthetic molecules bind to chloride ions, enveloping them in a ‘blanket of fat’ and making them easily soluble in bacterial membranes, taking the ions along with them and allowing them to function normally.” Disturbs the ion balance.” This study is a great example of fundamental knowledge of chemical fundamentals that has implications for an unmet need in human health research. ”
Professor Kevin Kavanagh, microbiologist in Maynooth University’s School of Biology, commented: This research is an example of chemists and biologists working together to pioneer the development of new antimicrobial agents with great promise.”
Such results pave the way for the potential development of anion transporters as viable alternatives to currently available antibiotics, which is urgently needed as the problem of AMR continues to grow. This is what has been done.
Reference: “Strong antimicrobial effects induced by disruption of chlorine homeostasis” Luke E. Brennan, Lokesh K. Kumawat, Magdalena E. Piatek, Airlie J. Kinross, Daniel A. McNaughton, Luke Marchetti, Conor Geraghty, Conor Wynne , by Hua Tong, Oisin N. Kavanagh, Finbarr O’Sullivan, Chris S. Hawes, Philip A. Gale, Kevin Kavanagh, Robert BP Hermes, August 23, 2023. chemistry.
DOI: 10.1016/j.chempr.2023.07.014
This research was supported by Science Foundation Ireland’s Pharmaceutical Research Center (SSPC) and the Irish Research Council (IRC).
Source: scitechdaily.com