DNA methylation is a widely observed epigenetic modification in biological systems that serves diverse functions in transcriptional regulation, transposable element silencing, and innate immunity.
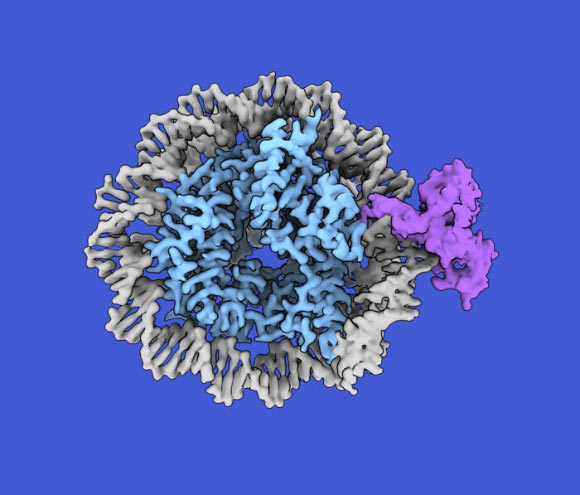
A nucleosome composed of DNA (grey) and histones (blue) with a single hemimethylated cytosine bound by CDCA7 (purple). Image courtesy of Kyohei Arita and Kazuaki Ushi.
DNA methylation is the process by which methyl groups are added to cytosine bases in DNA molecules and is the primary way in which DNA is epigenetically marked.
Epigenetic modifications act as on-off switches that regulate gene expression, helping to generate diverse cell types without altering the underlying DNA sequence – a way for the body to ensure that brain-related genes aren’t turned on in heart cells, for example.
Therefore, maintenance of DNA methylation patterns is crucial to ensure correct and consistent function of each cell type.
However, this is not easy: DNA methylation patterns can change over time, and this has been linked to a range of diseases.
One is a rare genetic disorder called immunodeficiency, centromere instability and facial anomalies (ICF) syndrome, whose symptoms include recurrent respiratory infections, facial abnormalities, and poor growth and cognitive function.
Although it was known that mutations in the CDCA7 gene cause ICF syndrome, little was known about the molecular function of this gene.
In a new study, Professor Hironori Funabiki of Rockefeller University and his colleagues have identified unique functional features of CDCA7 that ensure the correct inheritance of DNA methylation.
The researchers discovered that CDCA7 senses hemimethylation in eukaryotes, an important finding because hemimethylation sensing was long thought to be carried out exclusively by a protein called UHRF1.
“This is a really surprising discovery,” said Isabel Wassing, a scientist at Rockefeller University.
“The discovery that CDCA7 also acts as a sensor explains why mutations in it lead to diseases like ICF syndrome and fills a major gap in the field of epigenetics.”
“But it also raised new questions, such as why do cells need two different hemimethylation sensors?”
“We discovered that the CDCA7 gene, known to be the causative gene for ICF syndrome, specifically binds to hemimethylated DNA on nucleosomes and promotes DNA methylation by controlling the ubiquitination of histone H3,” said Atsuya Nishiyama, a research scientist at the University of Tokyo.
Scientists know that chromatin limits access for many enzymes and DNA-binding proteins, including those needed to introduce methylation into DNA.
Previous research by Professor Funabiki’s team has shown that CDCA7 forms a complex with a protein encoded by the HELLS gene, mutations of which also cause ICF syndrome.
HELLS is a so-called nucleosome remodeller that can temporarily release DNA molecules from nucleosomes.
“We reasoned that the CDCA7-HELLS complex is important in helping cells overcome the barrier of condensed heterochromatin and make DNA molecules available for methylation deposition,” Professor Funabiki said.
“But there are many nucleosome remodelers that can expose DNA molecules in this way.”
“It remained a mystery to us why CDCA7-HELLS is the only nucleosome-remodeling complex directly linked to DNA methylation maintenance.”
“By showing that CDCA7 specifically recruits HELLS to hemimethylated DNA, we finally have an explanation.”
In this model, CDCA7 recognizes hemimethylated DNA in chromatin and recruits HELLS to the site, which acts as a nucleosome remodeler to slide nucleosomes and reveal the hemimethylated site to UHRF1.
The takeover of hemimethylation sensing indicates that CDCA7 is better at detecting hemimethylation in dense heterochromatin than UHRF1 and also explains why cells require two distinct sensors.
“For these sensors to detect hemimethylation, they need to bind directly and selectively to hemimethylated DNA substrates,” Dr. Wassing said.
“CDCA7 appears to perform its function independently while DNA is wrapped around the nucleosome. Without CDCA7, UHRF1 cannot recognize the hemimethylation signals within the nucleosome particle.”
“Our findings suggest that CDCA7 and HELLS promote DNA methylation through a mechanism distinct from de novo DNA methylation, and this is strengthened by our demonstration that the CDCA7 HMZF domain specifically recognizes hemimethylated CpGs, which are substrates for the maintenance DNA methyltransferase DNMT1,” said Dr. Nishiyama.
“ICF disease-associated mutations in the CDCA7 gene abolish hemimethylated DNA binding, supporting the functional importance of hemimethylation detection by CDCA7.”
This new understanding may help elucidate the underlying mechanisms of diseases resulting from methylation dysfunction.
In the future, the functions of hemimethylation sensors beyond maintaining DNA methylation will be explored.
“Because some chromosomal regions are known to maintain a hemimethylated state, their recognition by CDCA7 may play a broader role in gene regulation and chromosomal organization, which is a very intriguing possibility,” says Professor Funabiki.
“Our research lays the foundation for the development of new DNA methylation inhibitors and therapeutic drugs for ICF syndrome,” said Dr. Nishiyama.
“Therapies that artificially control CDCA7-dependent DNA methylation may be useful for preventing cancer and aging and extending healthy lifespan.”
of Survey results Featured in this month’s journal Scientific advances.
_____
Isabel E. Wassing others2024. CDCA7 is an evolutionarily conserved hemimethylated DNA sensor in eukaryotes. Scientific advances 10 (34); doi: 10.1126/sciadv.adp5753
This article is based on a press release from Rockefeller University.
Source: www.sci.news