A research team led by physicists at Argonne National Laboratory isolated the energetic motion of electrons while “freezing” the motion of the much larger atoms they orbit in a sample of liquid water.
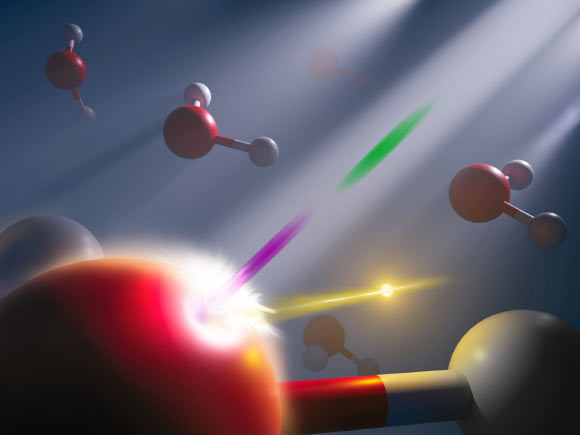
Shuai other. Synchronized attosecond X-ray pulse pairs (pictured here in pink and green) from an X-ray free electron laser were used to study the energetic response of electrons (gold) in liquid water on the attosecond time scale. On the other hand, hydrogen (white) and oxygen (red) atoms are “frozen” over time. Image credit: Nathan Johnson, Pacific Northwest National Laboratory.
“The radiation-induced chemical reactions we want to study are the result of targeted electronic reactions that occur on the attosecond time scale,” said lead author of the study, Professor Linda Young, a researcher at Argonne National Laboratory. said.
Professor Young and colleagues combined experiment and theory to reveal the effects of ionizing radiation from an X-ray source when it hits material in real time.
Addressing the timescales over which actions occur will provide a deeper understanding of the complex radiation-induced chemistry.
In fact, researchers originally came together to develop the tools needed to understand the effects of long-term exposure to ionizing radiation on chemicals found in nuclear waste.
“Attosecond time-resolved experiments are one of the major R&D developments in linac coherent light sources,” said study co-author Dr. Ago Marinelli, a researcher at the SLAC National Accelerator Laboratory.
“It's exciting to see these developments applied to new types of experiments and moving attosecond science in new directions.”
Scientists have developed a technique called X-ray attosecond transient absorption spectroscopy in liquids that allows them to “watch” electrons energized by X-rays move into an excited state before larger nuclei move on. “We were able to.
“In principle, we have tools that allow us to track the movement of electrons and watch newly ionized molecules form in real time,” Professor Young said.
The discovery resolves a long-standing scientific debate about whether the X-ray signals observed in previous experiments are the result of different structural shapes or motifs in the mechanics of water or hydrogen atoms.
These experiments conclusively demonstrate that these signals are not evidence of two structural motifs in the surrounding liquid water.
“Essentially, what people were seeing in previous experiments was a blur caused by the movement of hydrogen atoms,” Professor Young explained.
“By recording everything before the atoms moved, we were able to eliminate that movement.”
To make this discovery, the authors used a technique developed at SLAC to spray an ultrathin sheet of pure water across the pulse path of an X-ray pump.
“We needed a clean, flat, thin sheet of water that could focus the X-rays,” said study co-author Dr. Emily Nienhaus, a chemist at Pacific Northwest National Laboratory.
Once the X-ray data was collected, the researchers applied their knowledge of interpreting X-ray signals to recreate the signals observed at SLAC.
They modeled the response of liquid water to attosecond X-rays and verified that the observed signal was indeed confined to the attosecond timescale.
“Using the Hyak supercomputer, we developed cutting-edge computational chemistry techniques that enable detailed characterization of transient high-energy quantum states in water,” study co-authors from the University of Washington said Xiaosong Li, a researcher at Pacific Northwest National University. Laboratory.
“This methodological breakthrough represents a pivotal advance in our quantum-level understanding of ultrafast chemical transformations, with extraordinary precision and atomic-level detail.”
The team worked together to peer into the real-time movement of electrons in liquid water.
“The methodology we have developed enables the study of the origin and evolution of reactive species produced by radiation-induced processes encountered in space travel, cancer treatment, nuclear reactors, legacy waste, etc.,” Professor Young said. Stated.
The team's results were published in a magazine science.
_____
L. Shuai other. 2024. Attosecond Pump Attosecond Probe X-ray Spectroscopy of Liquid Water. science, published online on February 15, 2024. doi: 10.1126/science.adn6059
Source: www.sci.news