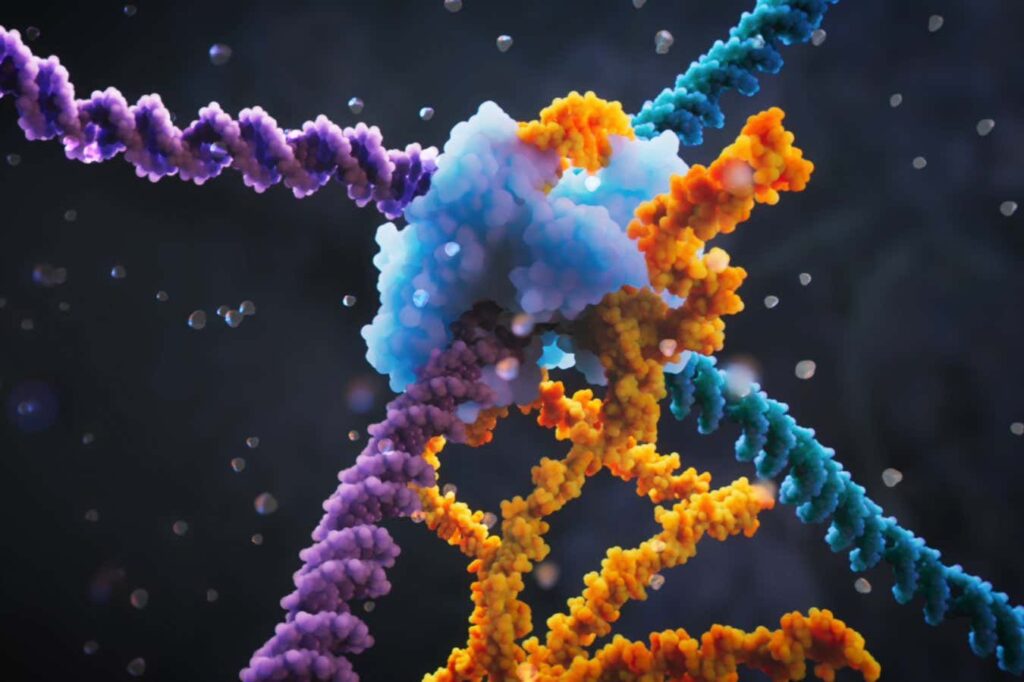
Bridge editing physically links two DNA strands
Visual Science
A powerful DNA-editing mechanism discovered in bacteria has the potential to enable much larger genome modifications than are currently possible with CRISPR-based techniques, but it is not yet clear whether it would work in human cells.
Patrick Shue Researchers at the Ark Institute in California call their new genome editing system the “bridge editing” system because it physically links, or bridges, two strands of DNA. “Using this system, we can modify large parts of the genome,” says Su. Su's team has figured out how bacterial “parasitic” DNA sequences naturally replicate using this system and how it can be applied to genome editing.
“We're excited about the possibility of making much broader genome modifications that go far beyond what we can do today with CRISPR,” he says, “and we see this as an important step toward a broader vision for genome design.”
CRISPR gene editing has revolutionized biology since it was introduced in 2012. It is used for a variety of purposes, and the first CRISPR-based therapeutic was approved last year. However, the basic form of CRISPR, which uses the Cas9 protein, is gene disruption rather than gene editing.
A standard CRISPR Cas9 protein has two parts: one part binds to a guide RNA molecule and looks for DNA that matches a specific section of the guide RNA. Custom guide RNAs are easy to create, so CRISPR Cas9 can be “programmed” to look for any part of the genome.
The second part of CRISPR Cas9 is a cutter that cuts the DNA when Cas9 binds to the target site. Once the cell has repaired the damage, Cas9 cuts it again. This action continues until an error is made during repair, resulting in the intended mutation of the target site.
While being able to mutate specific sites is useful, biologists want to make more precise changes, so they are modifying CRISPR proteins to edit DNA directly rather than relying on cellular repair mechanisms. For example, base editors can change one DNA letter to another without cutting the DNA, while prime editors can convert an extra section of guide RNA into DNA and add it to the target site.
These improved versions of CRISPR have the potential to treat a wide range of diseases, with several clinical trials already underway, but to address some diseases, more sophisticated genome modifications are needed. Many teams around the world are working on ways to achieve this. Some have realized that the mechanism by which genetic parasites cut and paste from one part of the genome to another, called IS110 elements, has potential because, like CRISPR, it is RNA-guided, but Hsu's team is the first to fully understand how it works.
The bridge-editing system consists of a so-called recombinase protein that binds to a guide RNA, such as the CRISPR Cas9 protein. What's unique about this system is that the guide RNA specifies two DNA sequences to seek out, not just one, Hsu's team found.
One sequence specifies the target site in the genome to modify, similar to CRISPR, and the other specifies the DNA to change. Using this system, DNA sequences of virtually any length can be added, deleted, or inverted.
There are already ways to do this, but they typically require multiple steps and leave behind a piece of extra DNA called a scar. “Bridge editing leaves virtually no scar,” Hsu says. “It offers an unprecedented level of control in engineering the genome.”
This means that it could be used for more than just replacing faulty genes, he says: It could also be useful for completely remaking the genomes of plants and animals. “What we want to do is go from inserting individual genes to doing chromosome-scale genome engineering,” Su says.
“The findings reported are certainly exciting and the underlying biology is truly surprising.” Steven Tang Bridge editing is being done at Columbia University in New York, but so far it has only been demonstrated to work in bacterial cells or in test tubes. Tang says it remains to be seen whether and to what extent bridge editing will work in complex cells like humans. But even if bridge editing doesn't work in initial tests in human cells, it may be possible to modify the system to work over time.
topic:
Source: www.newscientist.com