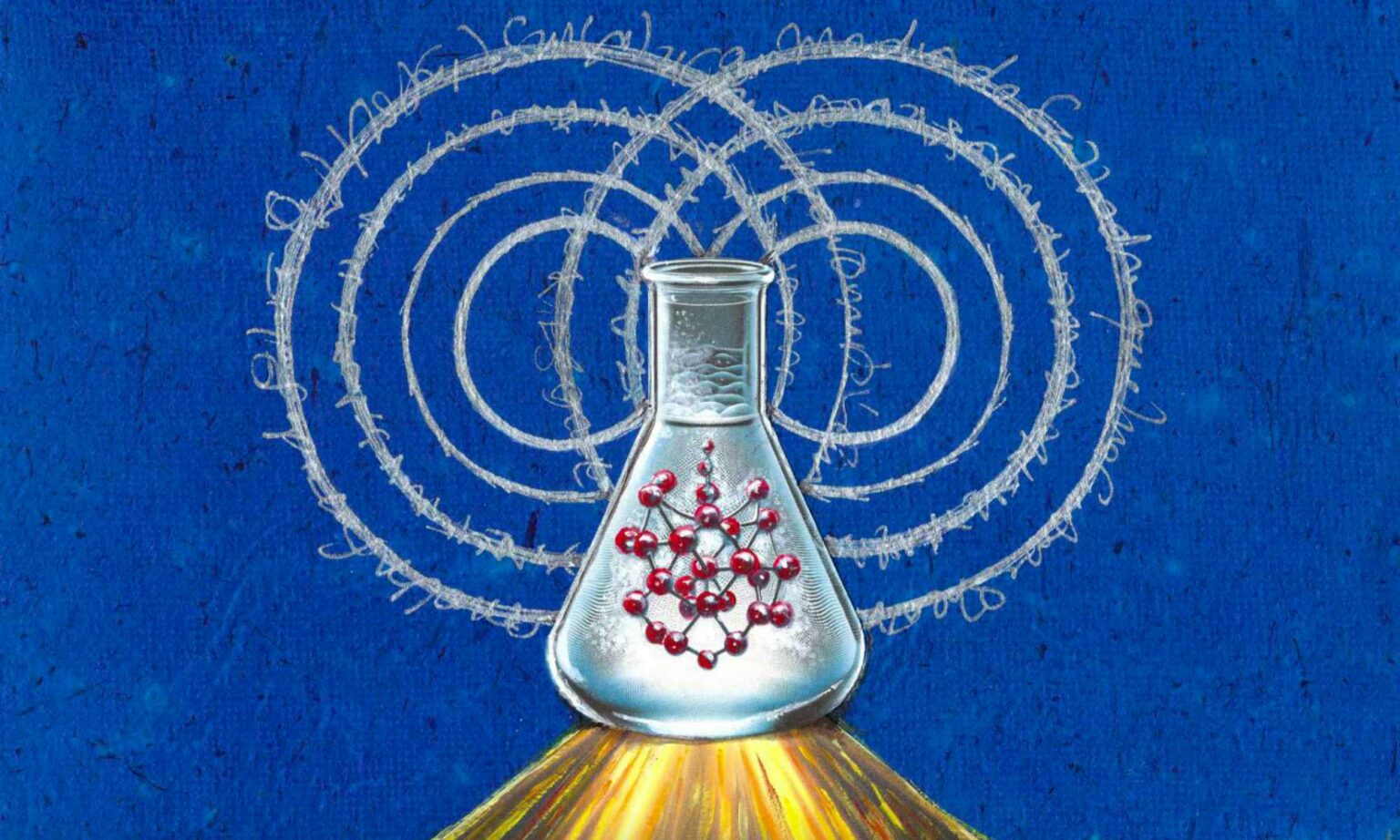
Rochester researchers have reported a strategy for understanding how molecules in completely chemically complex solvents lose their quantum coherence. This discovery opens the door to rational tuning of quantum coherence through chemical design and functionalization.
Credit: Annie Ostau de Lafon
This discovery can be used to design molecules with custom quantum coherence properties, laying the chemical basis for new quantum technologies.
In quantum mechanics, particles can exist in multiple states at the same time, which defies the logic of everyday experience. This property, known as quantum superposition, is the basis for new quantum technologies that promise to transform computing, communications, and sensing. However, quantum superposition faces a serious challenge: quantum decoherence. During this process, interaction with the surrounding environment disrupts the delicate superposition of quantum states.
Quantum decoherence challenges
To unlock the power of chemistry and build complex molecular architectures for practical quantum applications, scientists need to understand and control quantum decoherence so they can engineer molecules with specific quantum coherence properties. must be. To do so, we need to know how to rationally modify the chemical structure of molecules to modulate or alleviate quantum decoherence. To do this, scientists need to know the “spectral density,” a quantity that summarizes the speed at which the environment moves and the strength of its interactions with the quantum system.
A breakthrough in spectral density measurement
Until now, quantifying this spectral density in a way that accurately reflects molecular complexity has remained difficult in theory and experiment. However, a team of scientists has developed a way to extract the spectral density of molecules in a solvent using a simple resonance Raman experiment, a method that fully captures the complexity of the chemical environment.
A team led by Ignacio Franco, an associate professor of chemistry and physics at the University of Rochester, published their findings in Proceedings of the National Academy of Sciences.
Relationship between molecular structure and quantum decoherence
Using the extracted spectral density, we can not only understand how quickly decoherence occurs, but also determine which parts of the chemical environment are primarily responsible for decoherence. As a result, scientists can now map decoherence pathways and link molecular structure to quantum decoherence.
“Chemistry is built on the idea that molecular structure determines the chemical and physical properties of matter. This principle guides the modern design of molecules for medical, agricultural, and energy applications.” Using our strategy, we can finally begin to develop chemical design principles for emerging quantum technologies,” said Ignacio Gustin, a chemistry graduate student at the University of Rochester and lead author of the study.
Resonant Raman experiments: an important tool
The breakthrough came when the team realized that resonance Raman experiments provided all the information needed to study decoherence in its full chemical complexity. Although such experiments are routinely used to study photophysics and photochemistry, their usefulness for quantum decoherence had not been evaluated. The key insight was shared by David McCamant, an associate professor in the Department of Chemistry at the University of Rochester and an expert in Raman spectroscopy, and Jang Woo Kim, currently on the faculty at Chonnam National University in South Korea and an expert in quantum decoherence. This became clear from the discussion. He was a postdoctoral fellow at the University of Rochester.
Case study: Thymine decoherence
The researchers used their method to show for the first time how the superposition of electrons in thymine, one of the building blocks of humans, occurs. DNA, it takes only 30 femtoseconds (one femtosecond is one billionth of a billionth of a second) after absorbing ultraviolet light. They found that some vibrations within the molecule were dominant in the early stages of the decoherence process, while the solvent was dominant in the later stages. Furthermore, they found that chemical modifications to thymine significantly altered the decoherence rate, with hydrogen bonding interactions near the thymine ring resulting in more rapid decoherence.
Future implications and applications
Ultimately, the team’s research paves the way to understanding the chemical principles governing quantum decoherence. “We are excited to use this strategy to finally understand quantum decoherence in molecules of full chemical complexity and use it to develop molecules with robust coherence properties.” Franco said.
Reference: “Mapping the intramolecular electron decoherence pathway” by Ignacio Gustin, Chan Woo Kim, David W. McCamant, and Ignacio Franco, November 28, 2023. Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.2309987120
Source: scitechdaily.com