Diagram of interacting thick and thin filaments within cardiac sarcomeres based on structural cryo-electron tomography data. Credit: MPI of Molecular Physiology
Scientists have captured the first true-to-life 3D images of the thick filaments of a mammal’s heart muscle.
Atrial fibrillation, heart failure, and stroke are among the serious health conditions that can result from hypertrophic cardiomyopathy and are important factors in sudden cardiac death in people under 35 years of age.
“The heart muscle is the central engine of the human body. Of course, if you know how engines are made and how they work, it’s easy to repair a broken engine,” says Stefan Lunser. say. “At the beginning of our study of muscle, we were able to use cryo-electron microscopy to visualize the structure of key muscle components and how they interact.”
“But these were still images of proteins taken from living cells. We just don’t teach them much,” Rounser said.
through thick and thin
Skeletal and cardiac muscles contract through the interaction of two types of parallel protein filaments (thin and thick) within the sarcomere. Sarcomeres are subdivided into several regions called zones and bands, and these filaments are arranged in different ways.
Thin filaments are composed of F-actin, troponin, tropomyosin, and nebulin. Thick filaments are formed by myosin, titin, and myosin-binding protein C (MyBP-C). The latter can form bonds between filaments, while the so-called motor protein myosin interacts with thin filaments to generate force and muscle contraction.
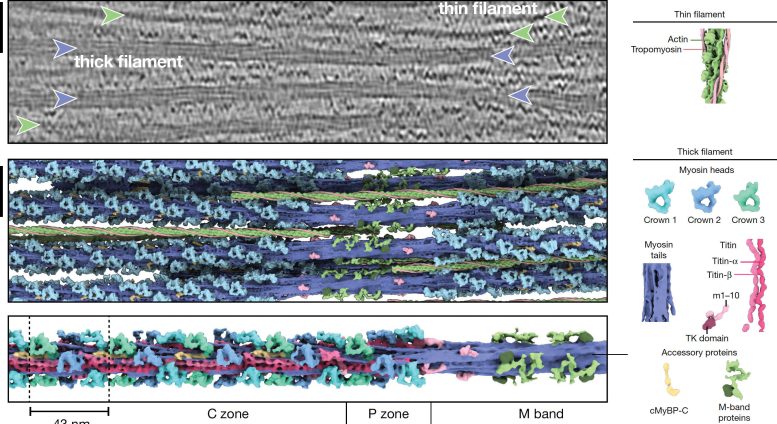
Thick filament structures within relaxed cardiac sarcomeres. The image above shows a tomographic slice of a cardiac sarcomere. Thin filaments are marked with green marks, thick filaments with purple arrows. The middle image shows reconstructed thick filaments (purple) and thin filaments (green). The image below shows the structure of thin filaments spanning several sarcomere regions. Scale bar indicates 50 nm. credit:
Molecular Physiology MPI
Muscle research milestones
“If we want to fully understand how muscles work at the molecular level, we need to delineate their components in their natural environment. This is one of the biggest challenges in biological research today. and cannot be addressed using traditional experimental approaches,” says Rounser.
To overcome this obstacle, his team developed an electron cryo-tomography workflow specifically for examining muscle samples. The scientists flash-frozen mammalian heart muscle samples produced by his Gautel group in London at very low temperatures (-175°C). ).
3D structure of a sarcomere showing thick filaments (purple) and thin filaments (green). Credit: MPI of Molecular Physiology
This maintains moisture and microstructure, keeping it pristine. Next, a focused ion beam (FIB milling) is applied to thin the sample to a thickness of approximately 100 nanometers, ideal for transmission electron microscopy, and multiple images are acquired while tilting the sample along its axis. Masu. Finally, computational methods reconstruct his three-dimensional image in high resolution.
In recent years, Raunser’s group has successfully applied customized workflows and recently published two groundbreaking publications. They created the first high-resolution images of sarcomeres and, so far, a misty muscle protein called nebulin. Both studies investigated the 3D organization of muscle proteins in sarcomeres, such as how myosin binds to actin to control muscle contraction, and how nebulin binds to actin to stabilize it and its We provide unprecedented insight into the 3D organization of muscle proteins in sarcomeres, including what determines their length.
complete the picture
In the current study, scientists have created, for the first time, high-resolution images of the heart’s thick filaments spanning several regions of the sarcomere. “With a length of 500 nm, this makes it the longest and largest structure ever resolved by cryo-ET,” said Davide Tamborini of MPI Dortmund, lead author of the study. Masu.
Even more impressive is the new insight gained into the molecular organization of the thick filaments and, by extension, their function. The arrangement of myosin molecules depends on their position within the filament.
Scientists believe that this allows the thick filaments to sense and process a large number of muscle-regulating signals and adjust the strength of muscle contractions depending on the sarcomere area. They also revealed how titin chains run along the filament. Titin chains intertwine with myosin and serve as a scaffold for its assembly, likely regulating length-dependent sarcomere activation.
“Our goal is to one day paint a complete picture of sarcomeres. The images of thick filaments in this study are ‘only’ snapshots of the muscle in its relaxed state. “We want to analyze sarcomeres in different states, such as during contraction, to fully understand how they function and how they are regulated,” says Rounser.
Comparisons with samples from patients with muscle diseases will ultimately contribute to a better understanding of diseases such as hypertrophic cardiomyopathy and the development of innovative treatments.
Reference: “Structure of native myosin filaments in relaxed cardiac sarcomeres” Davide Tamborrini, Zhexin Wang, Thorsten Wagner, Sebastian Tacke, Markus Stabrin, Michael Grange, Ay Lin Kho, Martin Rees, Pauline Bennett, Mathias Gautel, Stefan Raunser, 2023 October 32nd Nature.
DOI: 10.1038/s41586-023-06690-5
Source: scitechdaily.com












