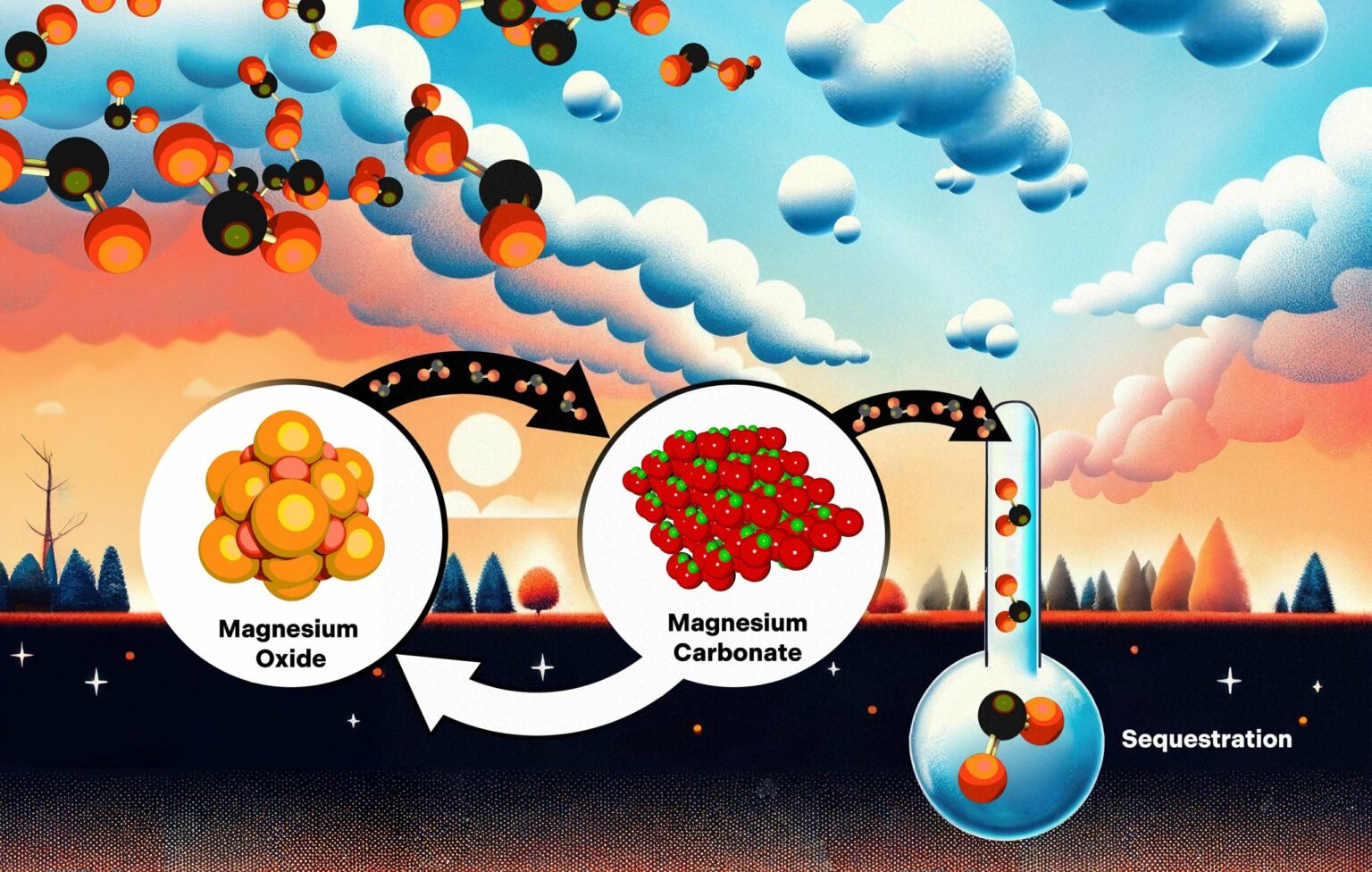
In the proposed carbon capture method, above-ground magnesium oxide crystals combine with carbon dioxide molecules from the surrounding air, causing the formation of magnesium carbonate. The magnesium carbonate is then heated back to magnesium oxide, releasing carbon dioxide and burying it underground or sequestering it.Credit: Adam Malin/ORNL, U.S. Department of Energy
A study of magnesium oxide for carbon capture by Oak Ridge National Laboratory revealed that the rate of absorption slowed over time due to the formation of a surface layer, posing challenges to economic viability and future This will guide research focused on solutions.
Magnesium oxide is a promising material for capturing carbon dioxide directly from the atmosphere and injecting it deep underground to limit the effects of climate change. However, for this method to be economical, we need to discover how quickly carbon dioxide is absorbed and how environmental conditions affect the chemical reactions involved.
Scientists at the Department of Energy’s Oak Ridge National Laboratory (ORNL) used samples of magnesium oxide crystals that had been exposed to the atmosphere for decades and those exposed for days to months to measure reaction rates. A set of magnesium oxide crystal samples were analyzed. They found that because a reactive layer forms on the surface of the magnesium oxide crystals, carbon dioxide is taken up more slowly over a longer period of time.
“This reaction layer is a complex mixture of different solids, limiting the ability of the carbon dioxide molecules to find fresh magnesium oxide to react with. To make this technology economical, we are currently , we are looking at ways to overcome this armor effect,” said ORNL’s Julian Weber, principal investigator on the project. Andrew Stack, an ORNL scientist and project team member, said: “If we can do that, this process could meet Earthshot’s carbon-negative energy goal of capturing gigaton levels of carbon dioxide from the air for less than $100 per metric ton of carbon dioxide.” ”
Most previous research aimed at understanding how quickly the chemical reaction between magnesium oxide and carbon dioxide occurs, relying on rough calculations rather than materials testing. The ORNL study marks the first time a decades-old test has been conducted to measure reaction rates over long periods of time. The researchers discovered the formation of a reactive layer using transmission electron microscopy at ORNL’s Center for Nanophase Materials Science (CNMS). This layer is composed of various complex crystalline and amorphous hydrate and carbonate phases.
“Additionally, by running computer simulations of reactive transport modeling, we found that as the reactive layer builds up, it becomes better able to block carbon dioxide from finding new magnesium oxide to react with,” ORNL researcher Vitaliy・Mr. Starchenko stated. “So in the future we’re looking at ways to circumvent this process and allow carbon dioxide to find new surfaces to react on.”
Computer simulations help scientists and engineers understand how reactive layers evolve and change the way materials move through them over time. Computer models enable predictions about how materials will react and move in natural and man-made systems, including materials science and geochemistry.
Reference: “Protection of MgO by a passivation layer prevents direct air capture of CO2” Juliane Weber, Vitalii Starchenko, Ke Yuan, Lawrence M. Anovitz, Anton V. Ievlev, Raymond R. Unocic, Albina Y. Borisevich, Matthew G. Bobinger and Andrew G. Stack, September 22, 2023 environmental science and technology.
DOI: 10.1021/acs.est.3c04690
The DOE Office of Science primarily supported this research. ORNL’s laboratory-directed research and development program supported time-of-flight (TOF), secondary ion mass spectrometry (SIMS), and preliminary transmission electron microscopy (TEM). His TOF-SIMS and TEM characterization using atomic force microscopy was conducted as part of a user project at CNMS, a user facility of the DOE Science Office of Science at ORNL.
Source: scitechdaily.com