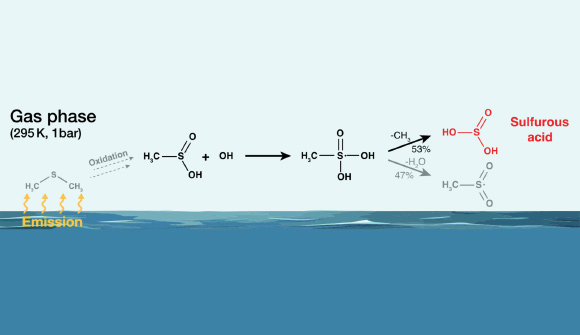
Chemists at the Leibniz Institute for Tropospheric Research have discovered that sulfurous acid (H2So3), once formed in the gas phase, is kinetically stable enough to allow its characterization and subsequent reactions.
In the gas phase, sulfurous acid, once formed, exhibits some kinetic stability with a lifetime of at least 1 second in atmospheric water vapor conditions. Image courtesy of Berndt others., doi:10.1002/anie.202405572.
Sulfurous acid Having formula H2So3 The molecular weight is 82.075 g/mol.
This molecule, also known as sulfuric acid(IV) or thioic acid, is a difficult-to-reach acid that has never before been observed in aqueous solution.
However, sulfite Detected It was discovered in the gas phase in 1988 by dissociative ionization of diethyl sulfite.
“The only experimental detection of sulfurous acid to date was achieved in 1988 by the team of Helmut Schwarz at the Technical University of Berlin using in situ generation with a mass spectrometer,” said Dr. Torsten Berndt of the Leibniz Institute for Tropospheric Research and colleagues.
“Under vacuum conditions, we estimated an extremely short lifetime of more than 10 microseconds.”
“Theoretical calculations show that H2So3 As a possible reaction product of the gas-phase reaction of OH radicals with dimethyl sulfide (DMS), which are produced from ozone and water molecules in the troposphere primarily in the presence of ultraviolet light.”
“DMS is produced primarily by biological processes in the ocean and is the largest source of biogenic sulfur in the atmosphere, producing approximately 30 million tonnes per year.”
The researchers experimentally investigated possible reaction pathways to H.2So3 It starts with DMS.
Formation of H2So3 Its formation in the gas phase was clearly demonstrated in a flow reactor under atmospheric conditions.
“Under our experimental conditions, sulfurous acid remained stable for 30 seconds, regardless of humidity,” the researchers said.
“With the existing experimental setup, longer residence times have not yet been explored.”
“Therefore, H2So3 It may persist in the atmosphere long enough to affect chemical reactions.”
“The observed yields were somewhat higher than theoretically expected.”
According to related model simulations, about 8 million tons of H2So3 They form every year all over the world.
“In this pathway, the mass of H increases by about 200 times.2So3 Sulfuric acid (H2So4“It produces carbon dioxide (CO2) from dimethyl sulfide in the atmosphere,” said Dr Andreas Tilgner and Dr Eric Hofmann from the Leibniz Institute for Tropospheric Research.
“The new results may contribute to a better understanding of the atmospheric sulfur cycle.”
Team paper Published in the journal Applied Chemistry.
_____
Torsten Berndt others2024. Gas-phase production of sulfurous acid (H)2So3) floats in the atmosphere. Applied Chemistry 63(30):e202405572;doi:10.1002/anie.202405572
Source: www.sci.news