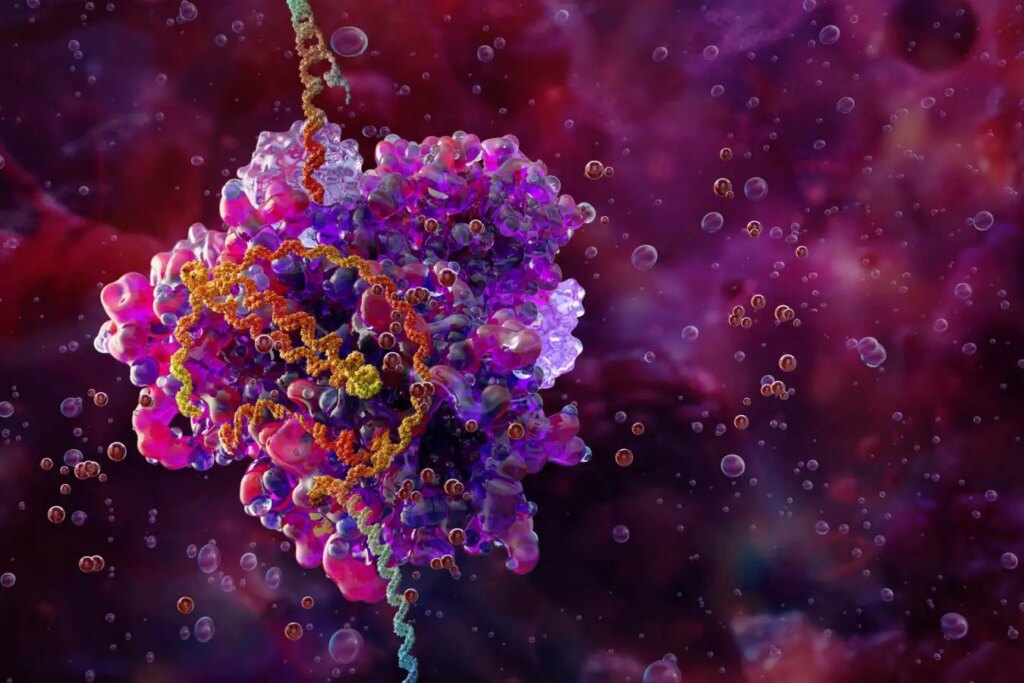
CRISPR-Cas9 Gene Editing Complex Diagram
Science Photo Library/Alamy
Imagine if postal workers could hand over flyers to volunteers on each block, who would then distribute them to neighbors. This approach could enable biologists to enhance gene editing for various medical conditions, significantly amplifying treatment effects.
The goal is to have each targeted cell replicate the gene editing machinery, subsequently passing it on to adjacent cells. This cascading effect can lead to transformative changes in the DNA of multiple cells, offering a breakthrough in treating genetic disorders.
In studies involving mice, Wayne Go and his team, including CRISPR pioneer Jennifer Doudna, successfully tripled the number of edited liver cells using this innovative method.
“We’re instructing the first cell to produce tiny lipid particles that carry the CRISPR machinery,” Ngo explained. “This transforms the cell into a factory, distributing these vital packets to surrounding cells.”
The first FDA-approved CRISPR treatment for sickle cell disease requires harvesting blood stem cells for editing outside the patient’s body, which makes it prohibitively expensive. However, many ongoing trials aim to develop methods for directly editing cells within the body, making treatments more accessible.
A major hurdle is delivering the CRISPR machinery to a significant portion of specific cells in the body. “To effectively cure sickle cell disease, about 20% of blood stem cells need to be edited,” Ngo noted. “Achieving that threshold has been challenging.”
Even if the initial delivery reaches only 10% of blood stem cells, local amplification could tip the scales to success by increasing that percentage to 30%.
To enable amplification, Ngo targeted proteins that assist in viral budding from cells. These proteins bind to cell membranes, forming small sacs or vesicles that can be transferred to other cells.
By linking the viral proteins to the CRISPR Cas9 editing protein, the Cas9 protein—which guides the gene editing process—can be encapsulated in vesicles and transported to neighboring cells.
In experimental tests, Ngo’s team injected a DNA sequence encoding the Cas9 viral protein into the livers of mice. Although only 4% of cells took up the DNA, they achieved a 12% overall gene edit rate.
Real-world applications of gene editing will utilize alternative delivery methods beyond pressure injection, which served only as proof of concept. “It’s not the most efficient method, but it demonstrates the potential of our system,” Ngo stated. “Tripling the amplification is a promising start, and we are actively exploring ways to refine our delivery systems to treat various diseases.”
This amplified gene editing approach not only enhances efficiency but could also allow for lower dosages, increasing treatment safety.
Researchers have been investigating vesicle budding strategies for many years. Gaetan Bourgeot of the Australian National University noted that Ngo’s team might be the first to validate these strategies in animal models for gene editing. However, Burgio emphasized the need for rigorous controls and validation of their results.
Current self-amplifying mRNA vaccines illustrate similar principles, where the delivered mRNA codes for mechanisms to produce more copies. This tactic aims to make vaccines safer and more cost-effective by reducing the required doses; however, the excess mRNA remains within the cells where it was produced.
Topics:
Source: www.newscientist.com